Abstract
The field of anti-cancer T cell therapy has had major advances in recent years with the development of the chimeric antigen receptor (CAR). Our group has developed and demonstrated that CARs against CD19 have complete remission rates of greater than 90% against B cell acute lymphoblastic leukemia (B-ALL). However, the progress against B-ALL has not been matched in other types of cancers. Acute Myeloid Leukemia (AML) is the most common acute leukemia in adults, presenting greater than 20,000 new cases per annum and representing 80% of acute leukemia. Additionally, prognosis is poor with as much as 70% of patients, 65 years or older, dying within 1 year of diagnosis. A recent study examining AML has shown that a TIM3 monoclonal antibody (mAb) blocks AML engraftment and eliminates leukemic stem cells (LSC). Additionally, TIM3 mAbs have anti-tumor effects such as slowing tumor progression. Gene-expression analysis demonstrates an 8-fold increase of TIM3 in LSC compared to hematopoietic stem cells. Our own evaluation of TIM3 expression on AML blasts and LSC demonstrated that TIM3 is commonly co-expressed on CD33+ blasts. These results led us to hypothesize anti-TIM3 CAR T cells can target and kill AML, which we will evaluate by the development of novel anti-TIM3 CARs. Generation of new CARs rely on a time consuming and/or costly process of phage display screening or hybridoma production, hybridoma screening, and immunoglobulin (Ig) sequencing. Here, we describe a novel CAR design strategy by next generation sequencing (NGS) followed by CAR synthesis and functionalvalidation against TIM3+ targets. NGS allows for direct identification of rearranged immunoglobulin genes, mutation and repertoire analysis, and comparison of multiple immunized mice. Furthermore, by eliminating the need for hybridoma production and screening we have developed a rapid, economical system for the development of novel CARs.
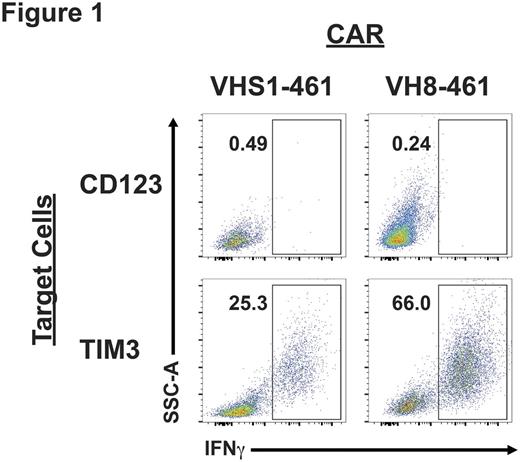
After immunization of four separate mice with CHO-TIM3, NGS demonstrated that nearly 70% of Ig heavy (IgH) and 61% of Ig light (IgL) sequences were accounted for by 2 and 3 V-J rearrangements, respectively. This suggested that these rearrangements were enriched for TIM3-reactivity and we designed pairs of these IgH and IgL rearrangements. By this process we generated 8 anti-TIM3 CARs (4 VHS1-based CARs and 4 VH8-based CARs). After their gene-synthesis and cloning into a genetic construct we produced retrovirus and transduced Jurkat, as well as primary T cells. Robust gene-transfer was observed with most constructs (14-64%). Furthemore, we detected antigen-specific cytokine production by Jurkat stimulated with TIM3+ targets (Fig. 1). We also examined in vitro killing by primary anti-TIM3 human T cells using a Real-Time cell Analysis cytotoxicity assay. We determined that anti-TIM3 CAR VH8-461 had the highest transduction efficiency, IFNγ production, and in vitro killing of target cells. We are now evaluating the in vivo efficacy of anti-TIM3 CAR T cells in immune deficient animal models implanted with TIM3+ targets. This work demonstrates NGS can be used to identify new sequences that can be developed rapidly and inexpensively into CARs. Using this process we created 8 anti-TIM3 CARs and by in vitro screening selected 1 of them (VH8-461) for in vivo validation. We are using this same NGS CAR generation system to develop additional anti-AML CARs which can be used in tandem with anti-TIM3 CARs to treat AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.